Author : James O’Siorain. Curtis Clock Lab, Royal College of Surgeons in Ireland.
Reviewed Article: Desynchronization of the molecular clock contributes to the heterogeneity of the inflammatory response.
Nancy C. Allen, Naomi H. Philip, Lucy Hui, Xu Zhou, Ruth A. Franklin, Yong Kong and Ruslan Medzhitov
DOI: 10.1126/scisignal.aau1851
Sci. Signal. 12 (571), eaau1851.
Many cells including immune cells such as macrophages exhibit a wide variety of responses to the same stimulus, even though we expect them to respond identically. As researchers we often call these different responses “background noise”. In the body, systemic time-rhythmic signals like cortisol synchronise cells, through their 24-hour molecular clock or circadian clock. However no systemic signals exist in culture, and as such circadian rhythmicity is lost. In this study, the authors investigated if this loss of circadian rhythmicity in culture may be contributing to background noise.
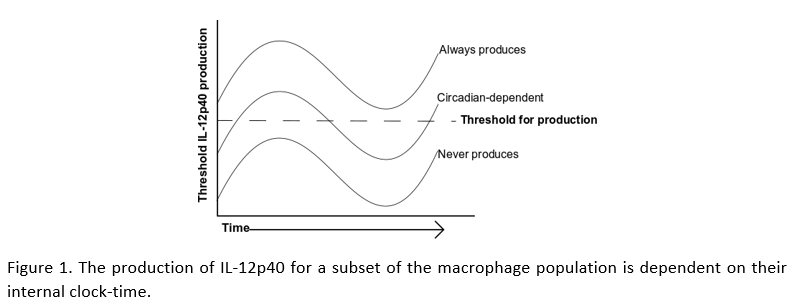
A frequent technique in immunology research is to treat macrophages with the bacterial component lipopolysaccharide (LPS), mimicking their response to a bacterial infection. Allen et al. isolated macrophages from mouse peritoneum at different times of day, stimulated with LPS, then used flow cytometry to see how the response changes across the whole population. They found that macrophages variably produce the inflammatory protein IL-12p40. Subsequent analysis demonstrated that IL-12p40 production was dependent on the clock-controlled genes, Nfil3 and Dbp. These genes have opposing effects on IL-12p40, with Nfil3 reducing, and Dbp enhancing the production of IL-12p40. While most macrophages readily produce IL-12p40, the production in others is hugely dependent on clock-time, while a further subset never produce IL-12p40.
These results are fascinating because they describe a threshold-of-activation for IL-12p40 production that is partially dependent on clock-timing. This reaffirms our understanding for the need of a balanced immune response and how disruption of our body clocks affects this balance. This study describes that the variability (or background noise) in macrophage responses to LPS, is partially dependent on NFIL3 and DBP abundance within each cell. However, there are many more ways by which the clock controls the inflammatory response. Circadian control over processes such as cell signaling, metabolism, and protein and nucleic acid modifications, likely contribute further to the heterogynous responses of the macrophage.